 Sarah Glenn and Dr. Godfrey
Sarah Glenn and Dr. Godfrey
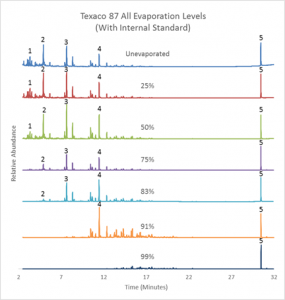
Due to the variety of different methods used to commit arson, identifying the accelerant used poses a challenge for investigators. In order to help combat this issue, gasoline samples taken from the Oxford, MS area were analyzed in order to understand the effect evaporation has on the gas chromatogram results of samples evaporated to predetermined levels using an internal standard of Di-n-Decyl Sulfide.
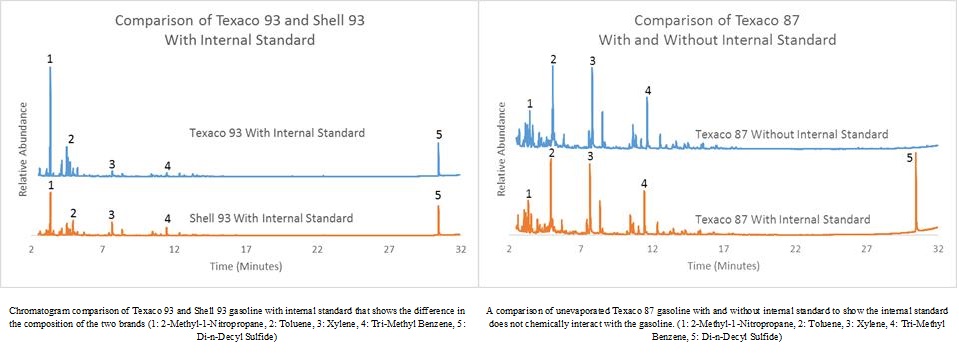
Three grades of gasoline from two different gasoline stations with octane ratings of 87, 89, and 93 with an added internal standard Di-n-Decyl Sulfide were analyzed using a Gas Chromatograph-Mass Spectrometer.
The use of an internal standard allows for the comparison of compound abundance in samples that can lead towards origin identification. The purpose of using two different gasoline stations is that each gas station uses a different makeup of gasoline during production, and analysis of the chromatograms can differentiate these differences.
This study confirms the hypothesis that by using the internal standard Di-n-Decyl Sulfide and the changes that occur in the gas chromatograms of gasoline samples that are evaporated, forensic chemists can assist arson investigators by determining fuel grades, sample origins (gas stations), and gasoline evaporation levels.